Quality Management System resolve
August 17, 2021MDR, IS AN EXTENSION THE RIGHT DECISION
December 19, 2022Simone Rudolph-Shortt Dec 2022
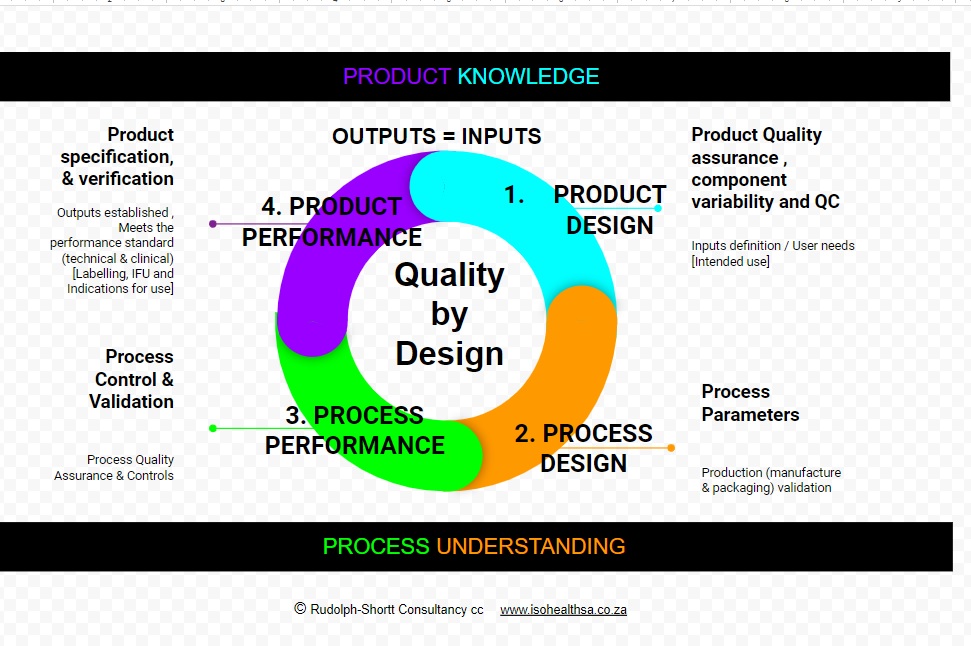
Quality by Design is defined as is a science and risk-based approach to ensuring consistent quality is achieved in a process or product. It is a systematic approach to development that begins with predefined objectives and emphasizes product knowledge and process understanding based on sound science and quality risk management. It is understanding the manufacturing process and identifying the key steps for obtaining and assuring a pre-defined final product quality. The company should constantly work to identify ways to improve the manufacturing process to ensure consistent product quality throughout the shelf life as well as to identify when contamination, non conformance or compliance or other production failures may occur. Improved quality by design will also lower product development and manufacturing costs by reducing the likelihood of production failures during a long run and by providing opportunities for continuous improvement. As part of its Quality by Design effort the company can work on three areas to support and increased manufacturing quality. The first is a continuous processing where materials constantly flow in and out of equipment. The second is the use of process analytical technology to monitor and control processes, as opposed to the current method of just testing products. The third is the development of new statistical approaches to detect changes in process or product quality. Ultimately Quality by Design” [QbD] is meant to incorporate quality into the product from the very beginning and achieve longstanding customer loyalty.